Synthetic Biology and Nanomedicine: Two AI Approaches for Removing Tumor Cells
Posted: April 4th, 2016 | Author: Domingo | Filed under: Artificial Intelligence | Tags: Dox, Engineered bacteria, FRET, Nanomedicine, Nanoporphyrin, Synthetic biology | Comments Off on Synthetic Biology and Nanomedicine: Two AI Approaches for Removing Tumor CellsResuming the topic of my post Multi-agent AI Nanorobots against Tumors, this time I’m going to explain two different AI approaches regarding the removal of tumor cells: firstly a synthetic biological approach in which E.coli bacteria are modified genetically in order to locate and eliminate tumor cells; and secondly a nanomedicine approach in which a polymer-based platform is used to remove carcinogenic cells.
Regarding synthetic biology, this is a discipline concerned with the design, construction, and modification of biomolecular systems and organisms to perform specific functions. Emerging applications of synthetic biology are amongst others the use of live bacteria as targeted delivery systems.
According to a study performed by a group of researchers of the University of California, bacteria can sense their environment, distinguish between cell types, and transmit proteins to eukaryotic cells. In this delivery situation it is paramount to control the interaction of a bacterium with a mammalian cell and to regulate it in response to environmental stimuli. The inv gene encoding invasin from Yersinia pseudotuberculosis represents a single-gene output interface for initiating adhesion and invasion of mammalian cells when expressed in E.coli. Inv+ E.coli can invade a broad range of tumor cells including epithelial, hepatocarcinoma, and osteosarcoma lines. The bacterial internalization can be synthetically linked to cell density, hypoxia, and inducible inputs.
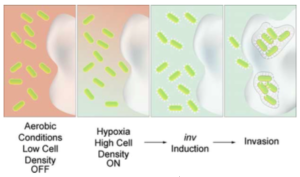
Fig. 1.- Design for Induction-Dependent Invasion of a Cancer Cell.
Above a critical cell density or in a hypoxic environment, sensors are activated resulting in the synthesis of invasin from Y. pseudotuberculosis and the invasion of tumoral cells. By restricting the expression of invasin to tumor sites, invasion could be confined to malignant cells. Specific invasion of tumor cells is only one component of an anti-cancer bacterium. Once inside target cells, a cytotoxic or immunostimulatory response must instigate destruction of the tumor. For this effect various bacteria must be engineered, which metabolize a chemotherapeutic prodrug at tumor sites.
Regarding the nanomedicine approach, nanoparticle-based agents are emerging as a promising paradigm. Several ‘soft’ organic nanoparticles -for example, paclitaxel (PTX)-loaded polymeric micelles (Genexol-PM), liposomal doxorubicin (Doxil), and PTX-loaded human serum albumin nanoaggregate (Abraxane)- have been approved or are currently in clinical trials for the treatment of human cancers, because of their excellent biocompatibility and drug-loading capacity.
In 2014 a group of North American and Chinese researchers developed a robust and smart porphyrinbased organic nanoconstruct (named nanoporphyrin, or NP). Blood is the first biological barrier for nanoparticle-based drug delivery systems via intravenous administration. Interaction with blood proteins and lipoproteins may cause the dissociation of nanoparticles and lead to premature drug release. In order to further increase the structural stability of NPs in blood circulation, the Chinese and North American researchers applied reversible disulphide cross-linking strategy to the NP platform (CNPs). These CNPs could also efficiently carry a variety of poorly water-soluble chemotherapeutic drugs and molecularly targeted drugs -for instance, DOX could be efficiently encapsulated inside CNPs.
The fluorescence resonance energy transfer (FRET) between the DOX and NPs was used to monitor the realtime drug release in human plasma. The FRET signal was very stable when CNPDOX was incubated in human plasma for 24h at 37°C, indicating that the DOX release from CNPs was slow. However, there was dramatic and immediate FRET signal decrease with each light exposure at 24 and 28h, indicating that CNPs could be triggered to release drug via illumination, probably as a result of local heat generation.
Therefore, these novel NPs can be used as nanocarriers with programmable releasing property that can minimize the premature drug release in blood and allow efficient release upon light irradiation and/or triggered by the endogenous reducing agent present at the tumor site or in cancer cells.
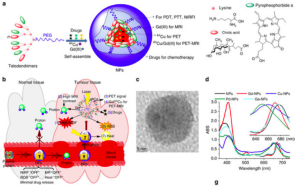
Fig. 2.- Design, Synthesis, and Characterizations of NPs
As a conclusion: engineered bacteria or nanoporphyrin platforms?
From my standpoint and according to what has been explained, it’s not necessary to go through that disjunctive stance: we can and should count on both approaches on fighting against cancer. The engineered bacteria and the integrated nanomedicine platform -by far less aggressive than the current medical treatments based on radiotherapy, chemotherapy, and surgical interventions- seem to show promising results and to raise high expectations as extremely versatile procedures against cancer: surely the hardest and toughest challenge ever faced by medicine.